
The first four of these are widely used, often in combination the last, on the other hand, is a highly radioactive and extremely rare substance. Known collectively by a term derived from a Greek word meaning "salt-producing," the halogen family consists of five elements: fluorine, chlorine, bromine, iodine, and astatine. Boca Raton, FL: CRC Press.Table salt, bleach, fluoride in toothpaste, chlorine in swimming pools -what do all of these have in common? Add halogen lamps to the list, and the answer becomes more clear: all involve one or more of the halogens, which form Group 7 of the periodic table of elements. CRC Handbook of Chemistry and Physics (84th ed.). The main issue with the heavier elements is their radioactivity. Toxicity decreases moving down the element group, with fluorine being the most toxic halogen and bromine the least toxic.

They can be toxic in pure form as diatomic elements. While several halogens are essential for life, the body uses them as ions. Exposure to tennessine would be dangerous because of its radioactivity. Humans and animals exposed to astatine accumulate it in the thyroid (like iodine), lungs, spleen, and liver. The are 10 to 20 milligrams of iodine in an average 70-kg person. Iodine is essential for human and animal nutrition, but serves no biological role in plants. A 70-kg human contains about 260 mg of bromine. No known biological role in humans is known, but a person consumes 1 to 20 milligrams of the element each day. A typical 70-kg human contains between 3 and 6 grams of fluorine.īromine occurs in all organisms. It’s possible trace amounts of this element are essential for human nutrition. The average 70-kilogram person contains about 95 grams of chlorine.įluorine is found in bones, teeth, hair, blood, urine, and eggs. Tennessine is not found in nature.Ĭhlorine is an essential element for plants and animals. Astatine is rare and not found in living organisms. Biological Roleįluorine, chlorine, bromine, and iodine all occur in the human body. Astatine and iodine isotopes find use in nuclear medicine. Iodine and bromine are used in halogen lamps, which glow with a whiter color than other incandescent lights. These two elements are widely used as flame retardants. Chlorine and bromine are disinfectants used to treat wounds, clean surfaces, and protect pools and spas. This makes the halogen group the only element group that contains all three normal states of matter at room temperature and pressure. Scientists predict tennessine is a solid. Fluorine and chlorine are gases at room temperature. The melting and boiling points of the halogens increase as you move down the periodic table.Similarly, the halogens display high electron affinities.Electronegativity decreases moving down the group on the periodic table. Fluorine is the most electronegative element. The halogens are highly electronegative.The group name “halogen” means “salt-producing” because the halogens react with metals to form salts. They readily bond with metals, particularly the alkali metals. Because of their electron configuration, the halogens are highly reactive.
HALOGEN PERIODIC TABLE FULL
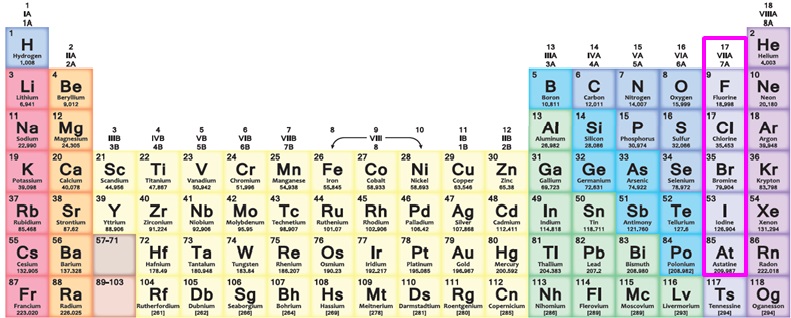
This is one less electron than needed for a full valence shell, so their usual oxidation state is -1. Atoms of halogen elements have seven valence electrons in their outer shell.They are poor conductors of heat and electricity and form brittle solids. The halogens share several common properties: Fluorine is too corrosive to be shown, while astatine is radioactive. Here are chlorine, bromine, and iodine (left to right) at room temperature. Here is a list of the halogens and a look at their properties, uses, and biological role. The halogens are group VII or 7 in older nomenclature and group 17 in modern IUPAC nomenclature.

They are found on the righthand side of the periodic table, just to the left of the noble gas group. The halogens are a periodic table group of elements. These elements are group 17 on the periodic table. The halogen elements are fluorine, chlorine, bromine, iodine, astatine, and tennessine. This entry was posted on Octoby Anne Helmenstine (updated on May 2, 2021)


 0 kommentar(er)
0 kommentar(er)
